
Leonard Richardson M.D.
President/CEO Kingdom Medicine Administrative Services Inc.
Picture Courtesy of NIAID-RML

Timeline
- December 31, 2019 – China CDC and WHO alerted for cluster of pneumonia – ruled out avian influenza, SARS etc
- January 7, 2020 – causative pathogen identified
- January 14, 2020 – First case in the US by date of illness
- January 23, 2020 – Chinese government limited movement Wuhan
- January 30, 2020 WHO declared this outbreak a Public Health Emergency of International Concern
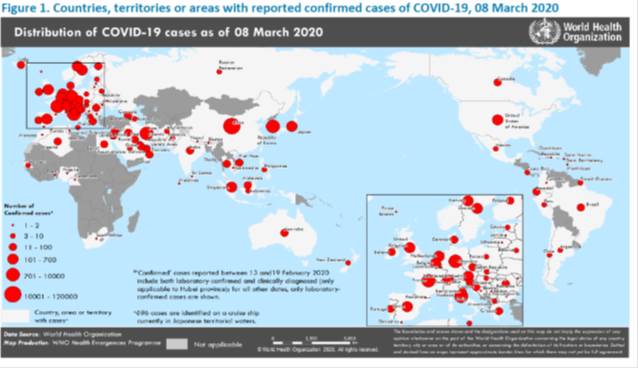
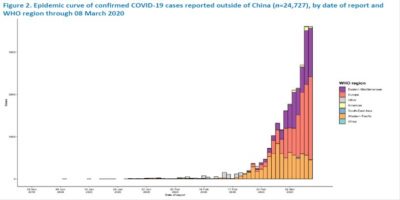
Eastern Mediterranean, Europe, Western Pacific
Epidemiology
- Incubation period – 5.6-7.7days travelers, 2-7days subset with known exposure, WHO report 5-6 days
- Severity – WHO report – 80% mild/asymptomatic, 13.8% severe, 6.1% critical
- Reproductive number – @ 2.2 -2.68
- Case fatality rate – China crude fatality rate 3.8% (17.3->0.7%), really 2%, lab confirmed 1.4%,
- Close contact transmission – Symptomatic – 10 pts, 445 close contacts active monitoring for sx 2/54 household contacts (10.5% of household contact), WHO report 3-10% household attack rate, 1-5% contacts, 0/11 HCW unprotected in Hong Kong no transmission
- Asymptomatic transmission – asymptomatic transmission case reports, serial interval 4.0-4.6 based on 20 pairs
Fauci NEJM 2020, Guan NEJM 2020, Nishiura IJID 2020, Backer Eurosurveillance 2020, Wu Lancet 2020, Tong EID 2020 Rothe NEJM 2020
https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf

COVID-19 Global Situation Summary
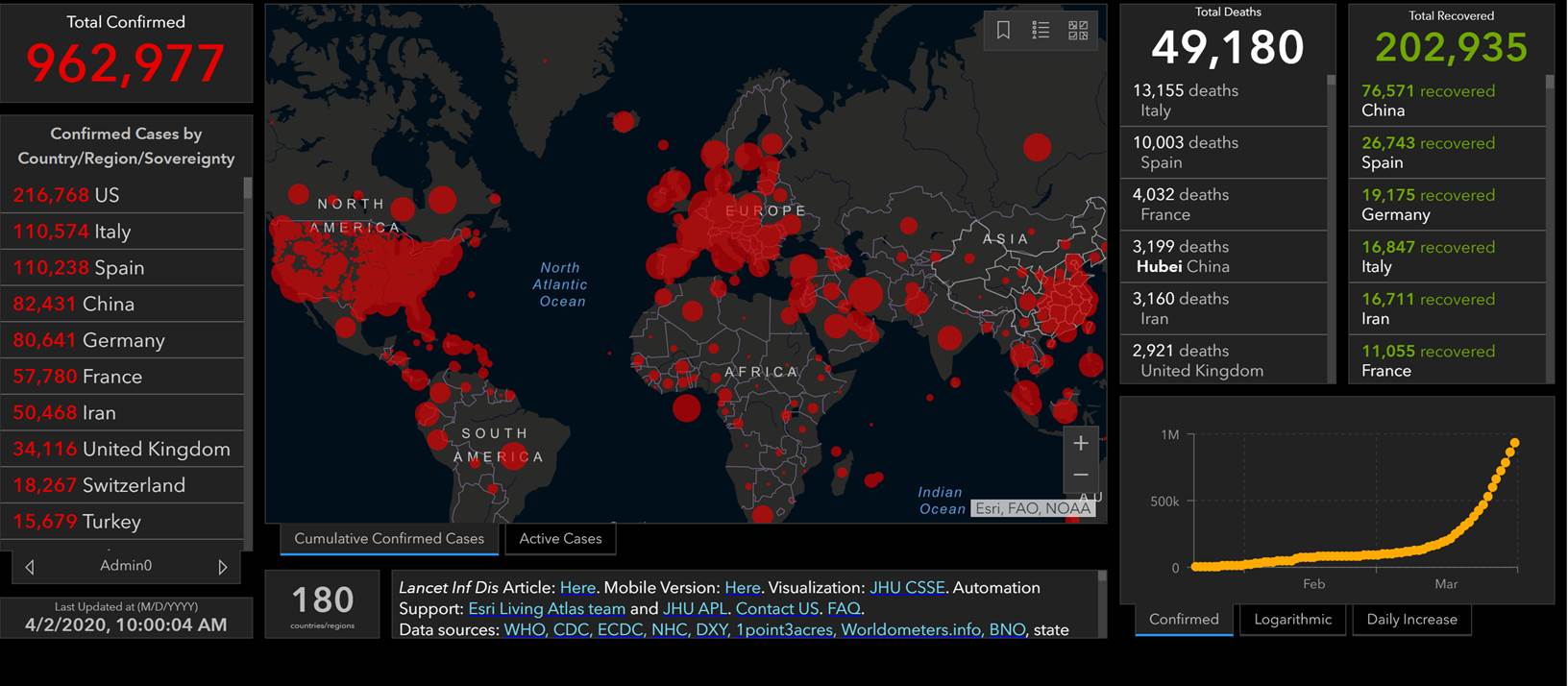
MDH prioritization
At this time, based on current local epidemiology, MDH is using the following criteria to prioritize testing at MDH:
- Person who had close contact with a laboratory-confirmed COVID-19 patient within 14 days of onset AND either fever or signs/symptoms of a lower respiratory illness
- Person with travel to a country with a CDC Level 2 or 3 Travel Health Notice or an area with confirmed ongoing community transmission within 14 days of onset AND has fever and signs/symptoms of a lower respiratory illness AND tested negative for influenza on initial work-up
- Person who resides in a nursing home or long-term care facility AND who has either fever or signs/symptoms of a lower respiratory illness AND who tested negative for influenza on initial work-up AND a respiratory virus panel negative for all pathogens AND no alternative diagnosis
U.S.: COVID-19 Cases
- 186,101 Confirmed Cases
- 1,110 Travel-related cases
- 3,128 Close Contact
- 181,863 “Under Investigation”
- 3,603 total deaths
- 55 jurisdictions
Source: www.cdc.gov, accessed April 2nd
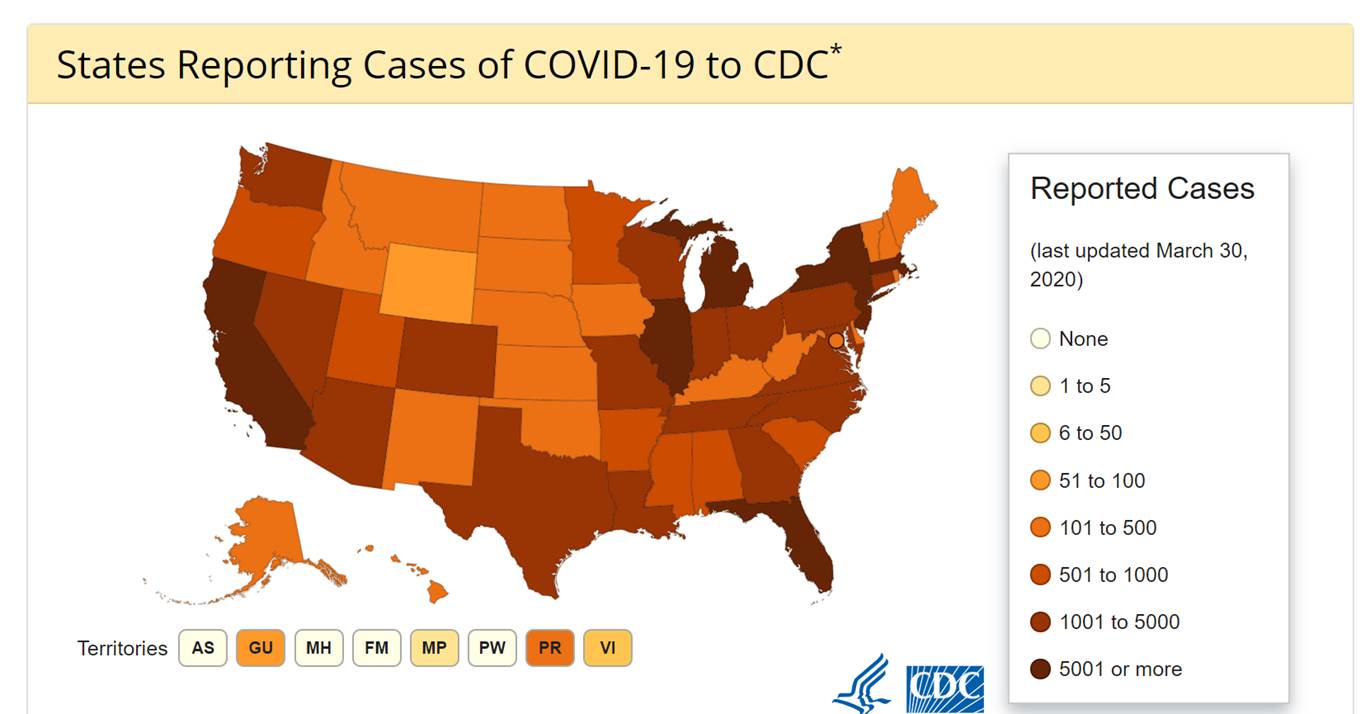
Maryland: COVID-19 Cases
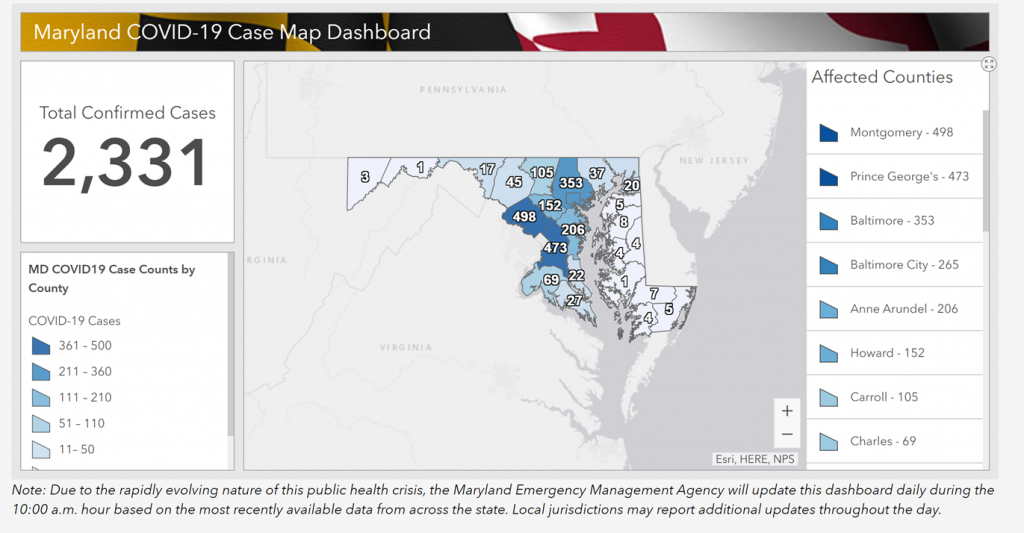
http://health.maryland.gov/coronavirus
Data current as of April 2nd, 2020
Maryland Epidemiology Breakdown
Cases by age range:
- 0-19: 55
- 20-39: 696
- 40-59: 908
- 60+: 672
Hospitalization: 26%
Deaths: 36
http://health.maryland.gov/coronavirus
Data current as of April 2nd, 2020
COVID-19 Testing in LTC
- Approval is NOT required from public health
- 1 Nasopharyngeal swab (NP) swab for COVID-19 testing
- Specimens can be safely collected in LTC using standard, contact, and droplet precautions (with a face mask) with eye protection
- Specimens should not be collected if a resident requires higher level of care
- Specimens should be sent to MDH laboratory to ensure rapid identification of residents with COVID-19
When should you be thinking about it?
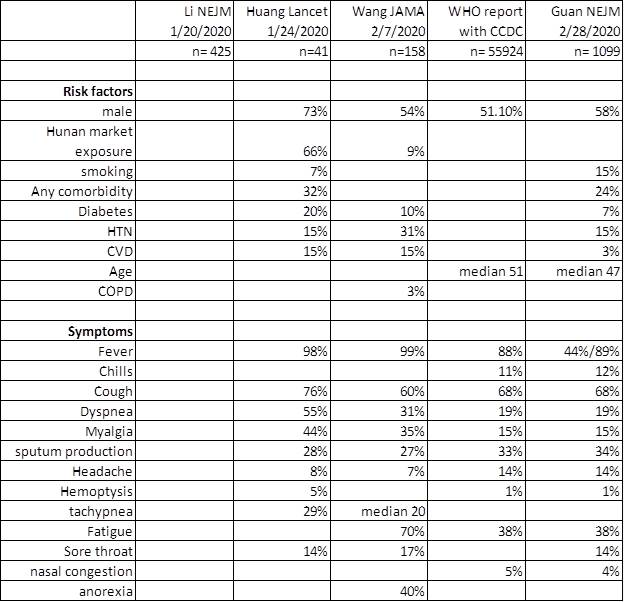
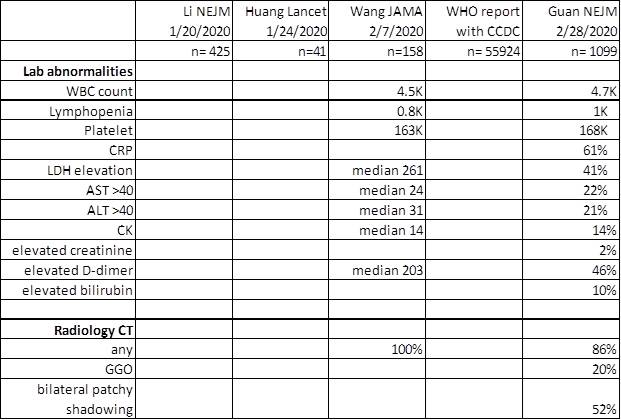
Fauci NEJM 2020, Guan NEJM 2020, Nishiura IJID 2020, Backer Euro surveillance 2020, Wu Lancet 2020, Tong EID 2020 Rothe NEJM 2020
https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
Laboratory testing
- RTPCR test
- MDH capacity
- JHH testing
- Commercial laboratory
New MDH Guidance – Preparing for and Responding to COVID-19
- New guidance document includes:
- Definitions for undiagnosed respiratory illness, suspect COVID-19, COVID-19 outbreak
- Requirements for reporting to public health
- Preventive measures against COVID-19
- Steps for responding to suspect COVID case(s)
- Steps for responding to positive COVID case(s)
New Universal Masking Recommendations

When COVID-19 cases are in the community, indicating community spread of COVID-19….
Universal masking should be considered
Universal Masking – The practice of wearing a face mask at all times while working within a facility.
How should universal masking be implemented:
- Staffing education – Staff should understand how to apply and remove face masks and when face masks should be changed.
- Ensure that staff understand that this is only ONE part of preventing the spread of COVID in LTC. Hand washing, appropriate use of PPE, not working while sick, and environmental cleaning are still important!
Mitigation vs. No Mitigation
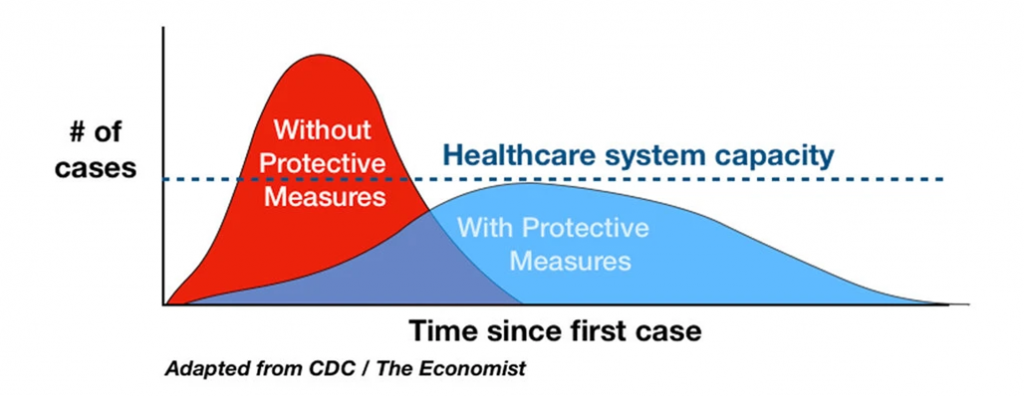
NYT 3/11/2020, adapted CDC/economist
P & I Mortality 1913-1917
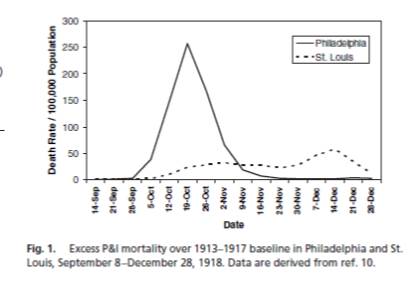
Hatchett PNAS 2007
Pandemic planning
-Keep minimally ill covid-19 patients outside hospital
-Reduce minimize elective visits/procedures – telehealth
-Operational capacity – workforce, space, covid-19 and non covid-19 supplies
-Social distancing
-Testing strategies
-Conserve supplies


HCP w/ COVID-19 – Return to work
Non-test-based strategy. Exclude from work until
At least 3 days (72 hrs) have passed since recovery defined as resolution of fever w/o the use of fever-reducing meds AND improvement in respiratory symptoms; AND,
At least 7 days have passed since symptoms first appeared
If HCP results are still pending, HCP may still return to work if criteria for non-test-based strategy are met
Return to Work
After returning to work, HCP should:
Wear facemask at all times in HC facility until sx fully resolved or until 14d after sx onset, whichever longer
Be restricted from contact w/ immunocompromised pts. until 14d after sx onset
Adhere to hand hygiene, respiratory hygiene, & other aspects of infection prevention and control
Self-monitor for sx and seek re-evaluation from occ health if respiratory symptoms recur or worsen
MDH Novel Coronavirus:
http://health.maryland.gov/coronavirus
MDH Laboratory Coronavirus:
https://health.maryland.gov/laboratories/Pages/Novel-Coronavirus.aspx
COVID-19 People at risk
https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html
CDC Coronavirus Prevention & Response in Long-term Care
https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/prevent-spread-in-long-term-care-facilities.html
CMS Coronavirus/LTC:
https://www.cms.gov/files/document/qso-20-14-nh-revised.pdf
CDC Guidance for Infection Control
https://www.cdc.gov/coronavirus/2019-nCoV/infection-control.html
Resources
 |
Questions? |
Email questions to MDH.IPCOVID@Maryland.gov
|



You must be logged in to post a comment.